Revolutionizing Influenza Vaccination: FDA Approves At-Home Nasal Spray Flu Vaccine
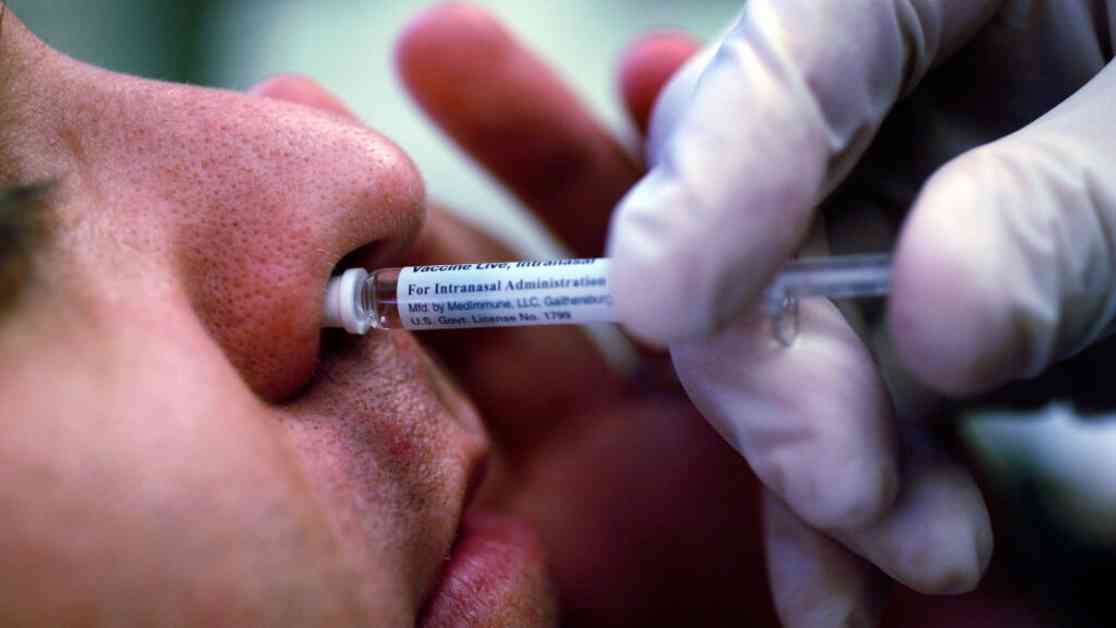
In a groundbreaking move, the Food and Drug Administration has given the green light to AstraZeneca’s nasal spray influenza vaccine, FluMist, for at-home administration. This decision marks a significant shift in how flu vaccines are delivered, offering individuals the option to self-administer the vaccine in the comfort of their own homes starting in the fall of 2025.
FluMist stands out as the only flu vaccine that is administered through nasal spray rather than an injection. It is specifically licensed for individuals aged 2 to 49, making it a convenient option for both children and adults. With this FDA approval, FluMist becomes the first flu vaccine in the United States that can be administered at home without the need for a healthcare professional.
Peter Marks, the director of the FDA’s Center for Biologics Evaluation and Research, emphasized the significance of this approval, stating, “Today’s approval of the first influenza vaccine for self- or caregiver-administration provides a new option for receiving a safe and effective seasonal influenza vaccine potentially with greater convenience, flexibility, and accessibility for individuals and families.”
Convenient Access to Flu Vaccination
While FluMist will still be available through traditional channels such as doctors’ offices and pharmacies for administration by healthcare providers, the option for at-home use brings a new level of accessibility to the vaccine. Individuals aged 18 and older can now order the vaccine through an online portal called FluMist Home, streamlining the process of receiving their flu shot.
To ensure the safety and eligibility of individuals opting for self-administration, a pharmacist will review an online questionnaire filled out by the individual. Once approved, the vaccine will be delivered directly to the individual’s home, making the vaccination process more convenient and efficient.
Iskra Reic, AstraZeneca’s executive vice president for vaccines and immune therapies, hailed the approval of FluMist for self-administration as a crucial advancement in expanding access to vaccines. This development is particularly significant in combating the annual burden of influenza and enhancing overall public health.
Managing Side Effects and Safety Concerns
As with any vaccine, it is important to be aware of potential side effects associated with FluMist. Reported side effects include fever over 100 degrees Fahrenheit in children aged 2 through 6, runny nose and nasal congestion in individuals aged 2 through 49, and a sore throat in adults aged 18 through 49.
By being informed about these potential reactions, individuals can better prepare for any temporary discomfort following vaccination. It is essential to consult with healthcare providers or pharmacists if there are any concerns about side effects or if additional information is needed regarding the administration of FluMist.
In conclusion, the approval of at-home nasal spray flu vaccination represents a significant milestone in public health efforts to combat influenza. By offering a convenient and accessible option for individuals to receive their flu shot, FluMist is poised to revolutionize the way flu vaccines are administered in the United States. With careful consideration of safety guidelines and potential side effects, individuals can now take proactive steps towards protecting themselves and their families from the seasonal flu.