Checkpoint Inhibitor from Incyte Shows Promise in Treating Anal Cancer
In a groundbreaking study presented in Barcelona, Spain, researchers have found that a checkpoint inhibitor developed by Incyte has shown significant potential in delaying disease progression in patients with a specific type of anal tumor. This exciting development could pave the way for the drug’s approval in treating a form of cancer that is predominantly caused by the human papillomavirus.
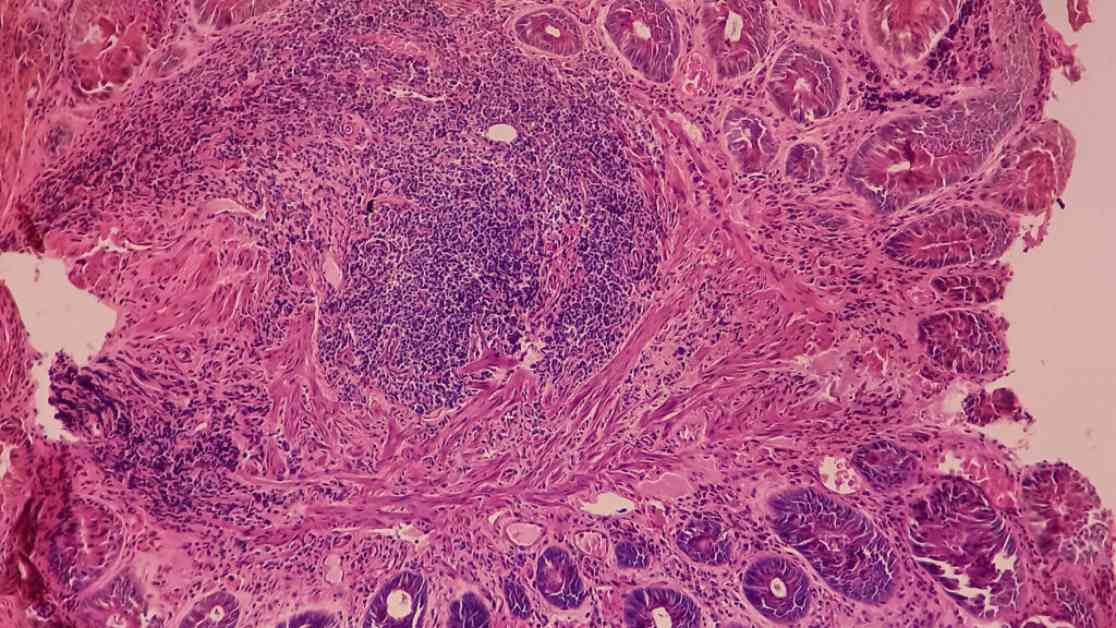
The Phase 3 trial focused on testing the PD-1 targeting antibody retifanlimab in combination with chemotherapy versus chemotherapy alone in patients with recurrent or metastatic squamous cell carcinoma of the anal canal. Retifanlimab, previously known as Zynyz in Merkel cell carcinoma, demonstrated promising results in the study.
The results of the study revealed that adding retifanlimab to chemotherapy reduced the risk of disease progression or death by more than a third. Patients who received the immunotherapy alongside chemotherapy went a median of 9.3 months without their disease progressing, compared to 7.4 months for those who only received chemotherapy. Additionally, individuals on the combination treatment appeared to have higher rates of overall survival, although further long-term data is required to confirm this advantage.
Subheadings:
1. The Potential of Immunotherapy in Anal Cancer Treatment
2. Study Results and Implications
3. Future of Checkpoint Inhibitors in Cancer Treatment
The Potential of Immunotherapy in Anal Cancer Treatment
Immunotherapy has revolutionized the field of oncology in recent years, offering new hope for patients with various types of cancer. In the case of anal cancer, which is often challenging to treat, the use of checkpoint inhibitors such as retifanlimab has shown promising results in delaying disease progression and improving overall survival rates.
The study conducted in patients with squamous cell carcinoma of the anal canal highlights the potential of combining immunotherapy with traditional chemotherapy to enhance treatment outcomes. By targeting the PD-1 pathway, retifanlimab helps the immune system recognize and attack cancer cells more effectively, leading to a delay in disease progression and potentially improved survival rates.
Study Results and Implications
The Phase 3 trial results presented in Barcelona have significant implications for the future of anal cancer treatment. The findings suggest that incorporating retifanlimab into standard chemotherapy regimens can significantly reduce the risk of disease progression and death in patients with recurrent or metastatic squamous cell carcinoma of the anal canal.
The data showing a median progression-free survival of 9.3 months in patients receiving the combination treatment compared to 7.4 months in those receiving chemotherapy alone is a promising indicator of the efficacy of retifanlimab in this patient population. Moreover, the observed trend towards higher overall survival rates in the combination group provides further support for the potential benefits of immunotherapy in treating anal cancer.
Future of Checkpoint Inhibitors in Cancer Treatment
The success of retifanlimab in delaying disease progression in anal cancer patients opens up new avenues for the use of checkpoint inhibitors in cancer treatment. As researchers continue to explore the mechanisms of action of these immunotherapies, there is growing optimism about their potential to improve outcomes for patients with various types of cancer.
Incorporating checkpoint inhibitors into standard treatment regimens could become a standard practice in the near future, offering patients a more targeted and effective approach to combating cancer. The findings from the Phase 3 trial of retifanlimab in anal cancer underscore the importance of continued research and development in the field of immunotherapy, with the goal of improving survival rates and quality of life for cancer patients worldwide.
In conclusion, the study presented in Barcelona represents a significant step forward in the treatment of anal cancer, demonstrating the potential of checkpoint inhibitors like retifanlimab to delay disease progression and improve overall survival in patients with squamous cell carcinoma of the anal canal. As researchers continue to explore the role of immunotherapy in cancer treatment, there is hope for a future where more effective and targeted therapies are available to patients facing this challenging disease.