Federal officials recently announced a breakthrough in telehealth prescriptions for stimulants and addiction treatment. The Drug Enforcement Agency (DEA) has extended the temporary rules that allowed health providers to prescribe drugs for opioid addiction and ADHD over telehealth until the end of 2025. This decision comes after months of debate among policymakers and stakeholders in the healthcare industry.
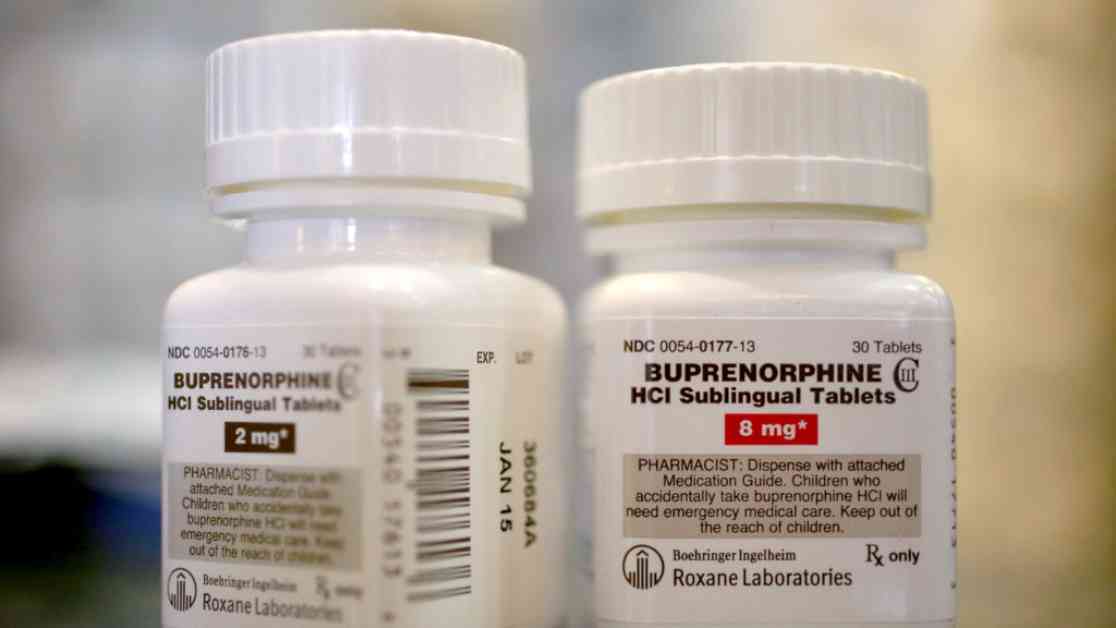
The pandemic brought about a significant shift in the regulations surrounding telehealth prescriptions for controlled substances like buprenorphine and Adderall. The temporary rules issued by the DEA allowed providers to prescribe these medications without an in-person visit, which was set to expire on January 1. With the extension until 2025, the DEA has postponed the resolution of this longstanding debate to the next administration.
The flexibility provided during the pandemic has sparked discussions about the barriers that strict regulations pose to accessing essential treatments. It has also been a boon for telehealth companies that have emerged to deliver care under the relaxed rules. However, the DEA’s draft rules released in 2023 faced criticism from telehealth advocates and providers, leading to an extension of the flexibilities until the end of this year.
The leaked draft rules proposed various restrictions, including the mandate that half of a provider’s controlled substance prescriptions must be written for patients seen in person, and the requirement to check patients against prescription drug monitoring programs in all 50 states. Telehealth companies raised concerns that these restrictions could force some services to close down. As a result, the DEA decided to extend the pandemic rules due to the lack of time to finalize new regulations by the end of the year.
While the extension aims to prevent fraudulent prescribing practices and ensure oversight, it has raised worries about potential setbacks in opioid addiction care. Companies offering online prescriptions for buprenorphine have been vocal about the need for continued protections for telehealth addiction care. The temporary rule underscores the importance of maintaining access to buprenorphine for individuals with opioid use disorder as a critical public health need.
Despite the celebration of the extension, stakeholders emphasize that it is only a temporary measure and more comprehensive solutions are required. Telehealth advocates argue that telemedicine is not just a temporary fix but a fundamental necessity in modern healthcare delivery. The extension of telehealth policies also includes considerations for Medicare enrollees to receive a broader range of services over telehealth for an additional two years.
In conclusion, the extension of telehealth prescriptions for stimulants and addiction treatment marks a significant development in healthcare policy. While it addresses immediate needs, stakeholders are pushing for more permanent solutions to ensure continued access to essential treatments through telehealth services. As the landscape of healthcare delivery evolves, the role of technology and telehealth will continue to shape the future of patient care.