FDA Approves Nasal Flu Vaccine for Home Use: What You Need to Know
The Food and Drug Administration (FDA) has recently approved a new nasal flu vaccine for home use, providing a convenient option for individuals to protect themselves against the flu virus. This development comes at a crucial time as the world continues to battle the ongoing COVID-19 pandemic, emphasizing the importance of preventive measures to safeguard public health.
Benefits of Nasal Flu Vaccine
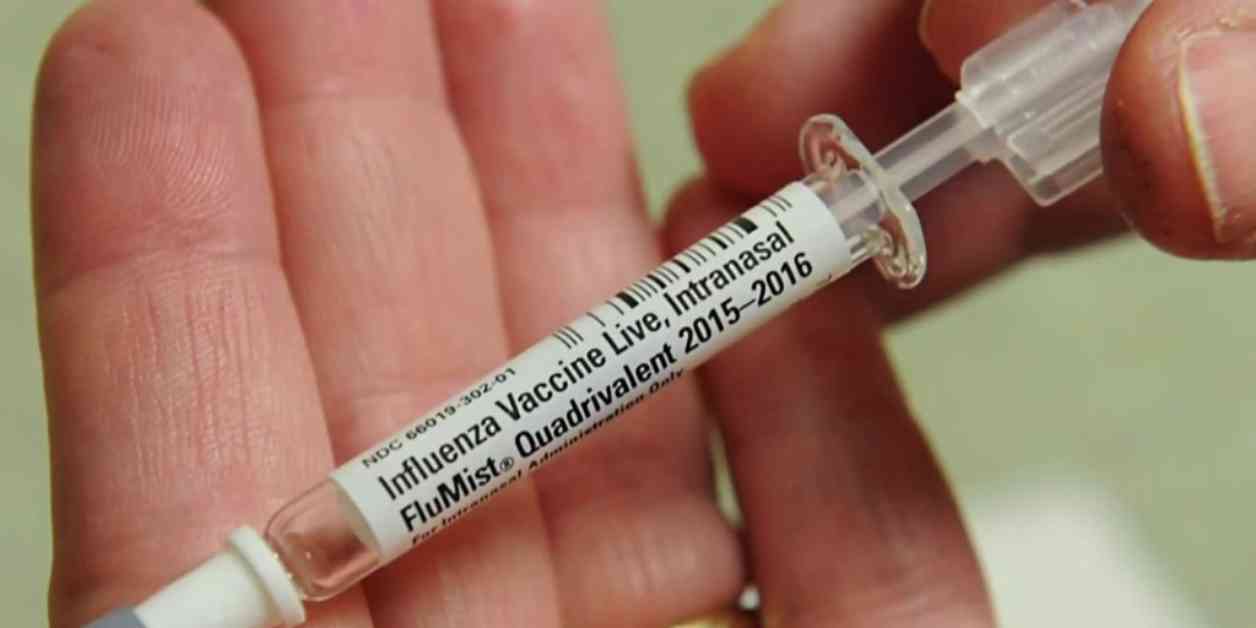
The nasal flu vaccine, also known as the live attenuated influenza vaccine (LAIV), offers several advantages over traditional flu shots. One of the key benefits is that it does not require a needle, making it a more appealing option for individuals who may have a fear of injections. This can be especially beneficial for children who may be hesitant to receive a flu shot.
Additionally, the nasal flu vaccine is administered through a nasal spray, which can be more comfortable and convenient for many people. This method of delivery may also result in a quicker immune response, potentially providing faster protection against the flu virus.
Effectiveness and Safety
It is important to note that the nasal flu vaccine has been shown to be safe and effective in preventing influenza. Clinical studies have demonstrated that the vaccine can significantly reduce the risk of contracting the flu virus, particularly in children and young adults.
Furthermore, the nasal flu vaccine has been found to be well-tolerated with minimal side effects. Common side effects may include mild nasal congestion, runny nose, or sore throat, which typically resolve on their own within a few days.
Availability and Administration
With the FDA’s approval, the nasal flu vaccine will now be available for home use, allowing individuals to administer the vaccine themselves without the need to visit a healthcare provider. This can be especially beneficial for individuals who may have difficulty accessing healthcare services or prefer the convenience of at-home vaccination.
It is important to follow the manufacturer’s instructions carefully when administering the nasal flu vaccine at home. Proper technique and storage conditions are essential to ensure the vaccine’s effectiveness and safety. Individuals should also consult with their healthcare provider if they have any questions or concerns about the vaccine.
In conclusion, the FDA’s approval of the nasal flu vaccine for home use offers a convenient and effective option for individuals to protect themselves against the flu virus. By taking proactive measures to prevent influenza, we can help reduce the burden on healthcare systems and protect vulnerable populations from serious illness. Stay informed, stay safe, and consider the nasal flu vaccine as part of your flu prevention strategy.