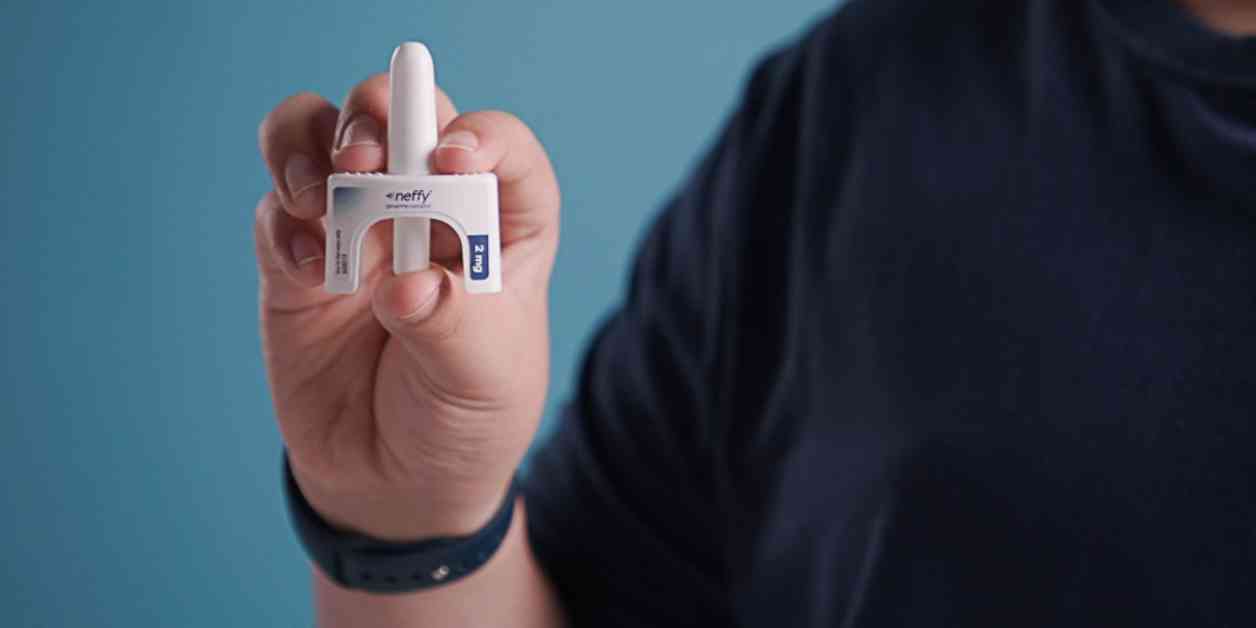
The Food and Drug Administration recently approved a new needle-free nasal spray from ARS Pharmaceuticals for the treatment of severe allergic reactions, including anaphylaxis. The spray, called Neffy, offers an alternative to traditional autoinjectors like the EpiPen.
Anaphylaxis is a serious and potentially life-threatening allergic reaction that requires immediate medical attention. Neffy is a single-dose nasal spray that is administered into one nostril. It has been approved for use in both adults and children weighing at least 66 pounds.
One of the key advantages of Neffy is that it eliminates the need for injections, which can be a source of fear and anxiety for some patients, especially children. By providing a needle-free option for administering epinephrine, Neffy may help reduce barriers to treatment and ensure that patients receive the care they need in a timely manner.
The approval of Neffy was based on several studies involving healthy adults, which demonstrated that the spray was effective in delivering epinephrine into the bloodstream. This evidence, along with the potential benefits of a needle-free treatment option, helped to secure the FDA’s approval for Neffy.
While the FDA initially had concerns about the spray and requested additional testing, the latest data and research have addressed these issues and demonstrated the safety and efficacy of Neffy. This approval marks an important milestone in the treatment of anaphylaxis and provides patients with a new and convenient option for managing severe allergic reactions.
Overall, the approval of Neffy represents a significant advancement in emergency allergy treatment and highlights the importance of innovation in the field of medicine. By offering a needle-free alternative to traditional treatments, Neffy has the potential to improve patient outcomes and ensure that individuals at risk of anaphylaxis have access to the care they need.