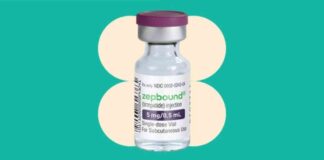
The Food and Drug Administration, or FDA, announced on Friday that it would be revisiting its decision to remove Eli Lilly’s tirzepatide, which is known as Mounjaro for diabetes and Zepbound for obesity, from its list of drugs in shortage. This decision comes after the agency had previously stated that the shortage of this popular drug had been resolved. As a result, compounding pharmacies were no longer allowed to produce and distribute generic versions of the drug.
However, many stakeholders, including compounders, telehealth companies, and patients, raised concerns about this decision. The Outsourcing Facilities Association, which represents FDA-registered compounding pharmacies, filed a lawsuit against the FDA in Texas. They argued that the drug was still in short supply and that the FDA’s actions were hasty and arbitrary. As a result, they sought a temporary restraining order to prevent the FDA from taking action against pharmacies that continued to produce generic versions of the drug.
This development represents a significant shift in the FDA’s approach to addressing drug shortages and regulating compounding pharmacies. It also highlights the complexities of the pharmaceutical industry and the challenges that arise when trying to ensure access to essential medications for patients.
The decision to reconsider removing tirzepatide from the shortage list indicates that the FDA is taking into account the concerns raised by various stakeholders. It also underscores the importance of balancing regulatory oversight with the need to ensure that patients have access to vital medications. As the situation continues to evolve, it will be crucial for all parties involved to engage in constructive dialogue and collaboration to find a sustainable solution.
In conclusion, the FDA’s decision to reevaluate the status of Eli Lilly’s tirzepatide is a significant development that will have far-reaching implications for patients, pharmacies, and the pharmaceutical industry as a whole. By responding to the concerns raised by stakeholders and actively engaging in dialogue, the FDA is demonstrating its commitment to addressing drug shortages in a thoughtful and inclusive manner.