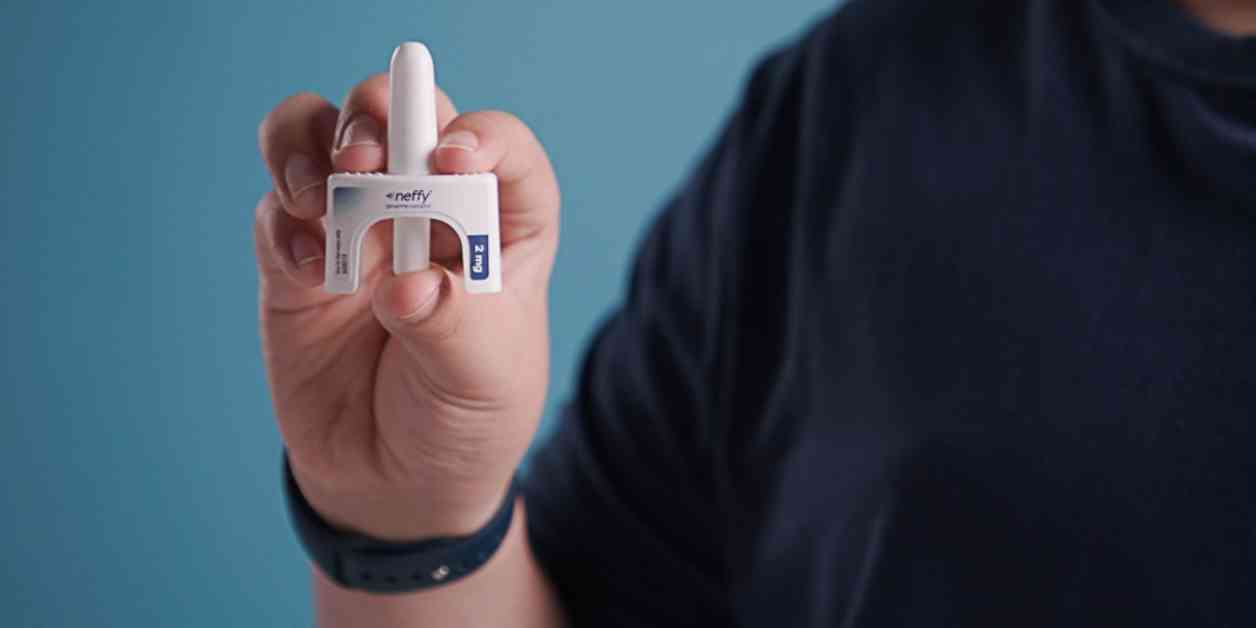
The Food and Drug Administration recently gave its approval for ARS Pharmaceuticals’ needle-free nasal spray to be used as an emergency treatment for severe allergic reactions, also known as anaphylaxis. This nasal spray, named Neffy, is considered a groundbreaking alternative to traditional autoinjectors like EpiPen and Auvi-Q, which require needles for administering epinephrine.
Anaphylaxis is a critical allergic reaction that affects multiple parts of the body and requires immediate medical attention. Neffy is a single-dose nasal spray that is administered into one nostril and has been approved for use in both adult and pediatric patients weighing at least 66 pounds. This approval is a significant step forward in providing a more accessible and user-friendly treatment option for individuals at risk of anaphylaxis.
Kelly Stone, an associate director at the FDA’s Center for Drug Evaluation and Research, highlighted that the fear of injections, especially among children, can often lead to delays in seeking treatment. With the introduction of Neffy, the barriers to quick and effective treatment for anaphylaxis may be reduced, ultimately saving lives.
The FDA’s decision to approve Neffy was supported by four studies involving 175 healthy adults. These studies focused on measuring the concentration of epinephrine in the blood after administering Neffy compared to traditional epinephrine injection products. The positive results from these studies led to the approval of Neffy for emergency use in cases of anaphylaxis.
It is essential to note that last year, the FDA initially declined to approve Neffy and requested further testing before granting its final approval. This cautious approach demonstrates the FDA’s commitment to ensuring the safety and efficacy of new medical treatments before they are made available to the public.
Overall, the approval of Neffy as a needle-free nasal spray for anaphylaxis treatment marks a significant advancement in emergency medical care for individuals with severe allergies. The accessibility, ease of use, and effectiveness of this new treatment option have the potential to make a positive impact on the lives of those at risk of anaphylaxis, providing them with a faster and more convenient way to receive life-saving medication during critical situations.