DEA Introduces New Telehealth Rules for Controlled Substances
The Drug Enforcement Administration (DEA) has finally unveiled a long-awaited announcement regarding the prescription of controlled substances through telemedicine. This groundbreaking move comes after a 16-year delay in implementing a mandate issued by Congress back in 2008. The new regulations, issued just before the end of President Biden’s term, are poised to reshape the landscape of telehealth services, particularly in the realm of prescribing medications like opioids and stimulants used to treat ADHD.
Implications of the New Regulations
Under the proposed rules, prescribers seeking to issue Schedule II medications, such as Ritalin and Adderall, via telemedicine would be subject to stringent guidelines. One notable restriction requires providers to be physically located in the same state as their patients and to conduct at least 50% of prescription appointments in person. These regulations have sparked immediate backlash from telehealth providers and industry organizations like the Alliance for Connected Care, who argue that such constraints undermine the core principles of virtual healthcare access.
Challenges and Opportunities in Telehealth
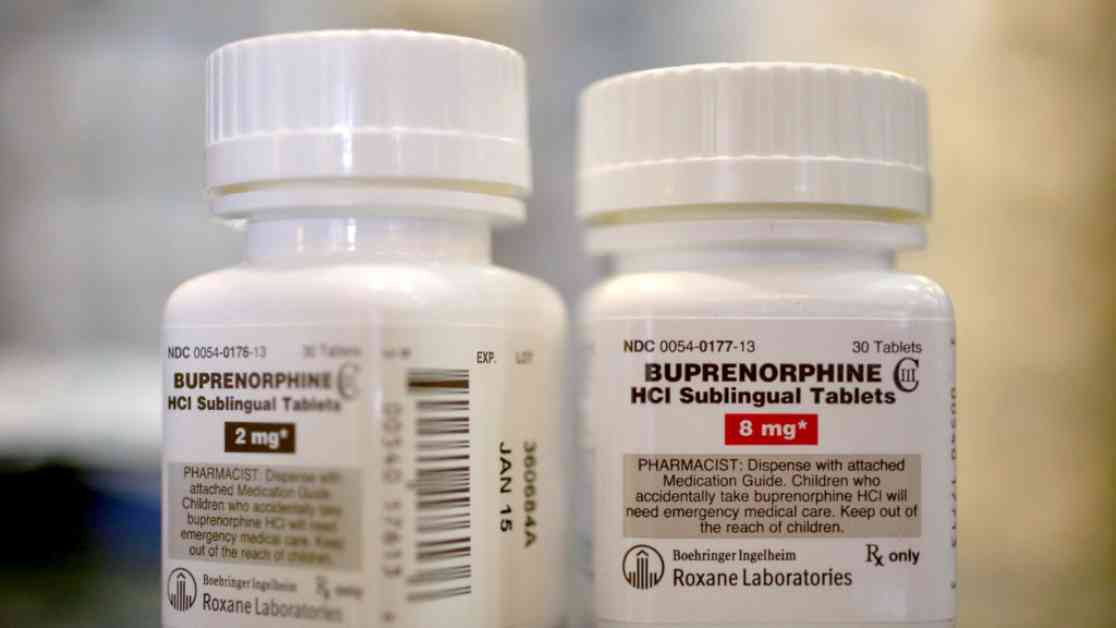
While emergency measures enacted during the Covid-19 pandemic granted temporary flexibility in telehealth prescribing, the DEA’s efforts to solidify post-pandemic regulations have encountered resistance. Of particular concern is the prescribing of buprenorphine, a Schedule III medication used to treat opioid addiction. The DEA’s decision to allow prescribers to issue six months’ worth of buprenorphine without an in-person visit marks a significant shift in telehealth prescribing practices.
Looking Ahead: The Future of Telehealth
As stakeholders grapple with the implications of the DEA’s new regulations, the future of telehealth remains uncertain. Providers continue to rely on the agency’s extension of Covid-era flexibilities, with timelines for finalizing regulations being a point of contention. The potential impact of these rules on patient care and access to essential medications via telemedicine looms large, underscoring the need for ongoing dialogue and collaboration among all involved parties.
In conclusion, the DEA’s move to establish a special registration process for telehealth prescribers represents a pivotal moment in the evolution of virtual healthcare delivery. As the healthcare landscape continues to evolve, navigating the intersection of regulation, technology, and patient care will be critical in shaping the future of telehealth services.