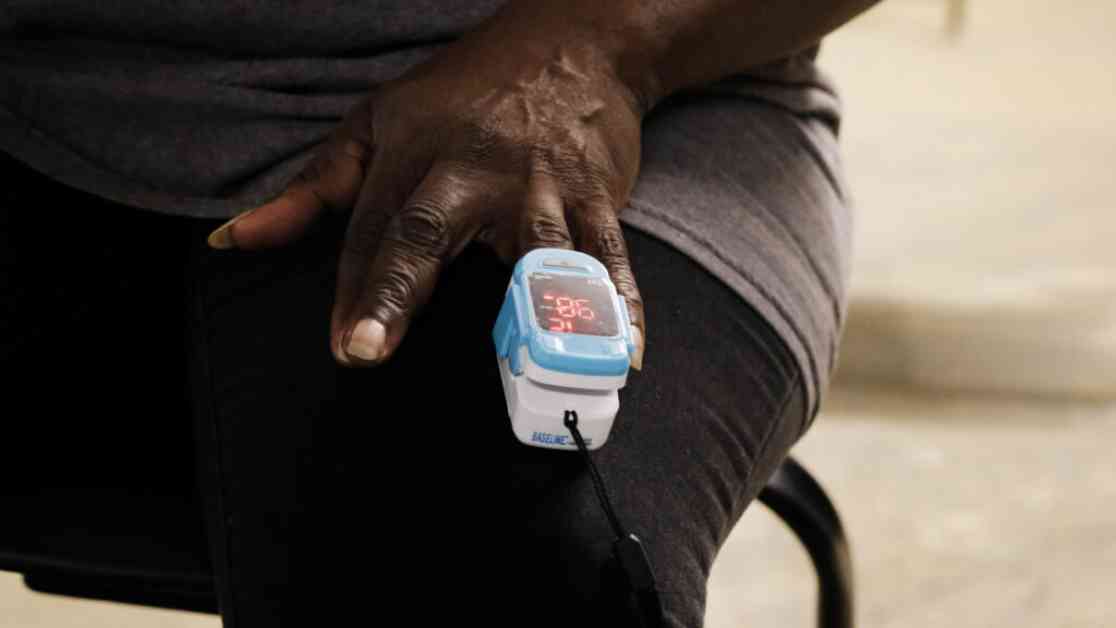
In 2022, Noha Aboelata, the CEO of a health clinic in Oakland, California, noticed a problem with pulse oximeters. These devices, used to measure blood oxygen levels, were found to be biased against Black and brown patients. The oximeters overestimated oxygen levels in people with darker skin, leading to inaccurate readings and potentially harmful consequences for patients.
To address this issue, Aboelata decided to take legal action against 12 pulse oximeter manufacturers and distributors. The lawsuit, filed nearly a year ago, has already resulted in settlements from companies like Medtronic. While the exact terms of the settlements remain undisclosed, Medtronic has taken steps to educate physicians about the limitations of their devices and provide QR codes that link to educational information on pulse oximetry.
While these developments are a positive step, experts like Sara Gerke emphasize that more needs to be done to create equitable healthcare products. Medtronic has also established a lab in Colorado to test future devices on a more diverse range of skin tones, aiming to improve accuracy for all patients.
The limitations of pulse oximeters, particularly in darker skin tones, have been highlighted during the COVID-19 pandemic. Health care workers struggled to assess the severity of Black patients’ cases due to inaccuracies in the devices. Calls for clearer labeling and rigorous testing of pulse oximeters have been made by senators, state attorneys general, and advocacy groups.
Despite these efforts, the FDA has yet to issue updated guidelines for pulse oximeters. The delay has frustrated healthcare professionals like Theodore J. Iwashyna, who have witnessed the impact of inaccurate readings on patient care. Aboelata’s clinic’s lawsuit in California has prompted some companies to settle and make changes to their devices, but there is still work to be done on a national level.
Roots Community Health, Aboelata’s clinic, has reached settlements with several companies, including Veridian Healthcare and Gurin Products. Medtronic, as a major player in the pulse oximeter market, has agreed to prioritize patient safety by providing information on potential inaccuracies in their devices. The hope is that changes made in California will set a precedent for the rest of the country.
Experts are eagerly awaiting updated FDA guidance on pulse oximeters, which they believe will lead to more accurate devices for all patients. While education is important for raising awareness of the issue, regulatory action is necessary to ensure that healthcare technologies work effectively for everyone. The FDA’s commitment to issuing new guidelines is a step in the right direction, but more progress is needed to achieve health equity.