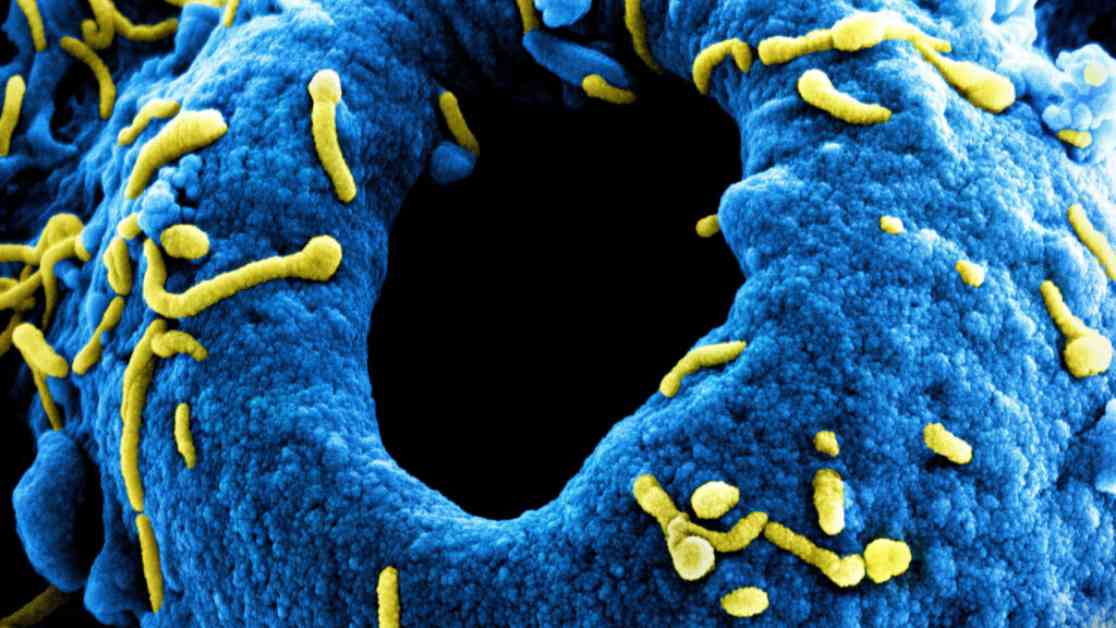
Experimental vaccines and treatments to combat the Marburg virus outbreak in Rwanda are set to arrive this weekend. These will be used in clinical trials that are expected to start soon. The Marburg virus is closely related to the Ebola virus and causes similar symptoms and transmission patterns. Although Marburg outbreaks are usually smaller, they have high fatality rates similar to Ebola.
Rwanda is facing its first Marburg outbreak with 41 confirmed cases, making it one of the largest on record. Twelve people have already died from the virus, with over 80% of cases among healthcare workers. The clinical trials are crucial, and the country has agreed to conduct them promptly.
Health Minister Sabin Nsanzimana mentioned during a press conference that the trials could begin within days, emphasizing the importance of saving the lives of healthcare workers. Questions remain about the design of the trials, particularly regarding the comparison of different vaccines or therapeutics.
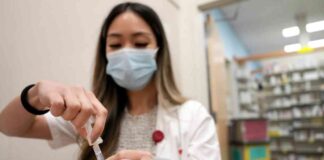
Two treatment options will be available in Rwanda – Gilead Sciences’ remdesivir and Mapp Biopharmaceutical’s MBP-091 monoclonal antibody. Remdesivir is already approved for COVID-19 treatment in the US and has been tested against Ebola. Meanwhile, the US government is supplying doses of the vaccine and monoclonal antibody developed with financial support from BARDA.
The Sabin Vaccine Institute is sending 700 doses of a single-dose vaccine for Marburg. This vaccine, developed at the National Institutes of Health’s Vaccine Research Center, is in a Phase 2 trial in Uganda and Kenya. Nancy Sullivan, a researcher involved in designing the vaccine, praised the WHO’s preparedness efforts to respond to outbreaks like Marburg quickly.
The Sabin Vaccine Institute’s CEO, Amy Finan, highlighted the collaborative efforts between the institute and the Rwandan government to combat the outbreak effectively. The quick response from Rwanda and the availability of vaccine doses have been crucial in addressing the situation promptly.
In conclusion, the arrival of experimental vaccines and treatments in Rwanda marks a significant step in combating the Marburg outbreak. The clinical trials and collaborative efforts between international organizations and the Rwandan government are essential in containing the spread of the virus and saving lives. This proactive approach and rapid response serve as a model for addressing future outbreaks of rare diseases effectively.