**Alzheimer’s Drug Linked to Increased Brain Bleed Risk in Down Syndrome Patients**
In a groundbreaking study published in JAMA, researchers have shed light on the potential risks associated with using anti-amyloid drugs in individuals with Down syndrome. The study, led by neurologist Lei Liu from Brigham and Women’s Hospital, found that people with Down syndrome may face a higher risk of brain hemorrhages and brain swelling when treated with drugs like lecanemab, which are designed to slow the progression of Alzheimer’s disease.
**Safety Concerns for Down Syndrome Patients**
The approval of lecanemab by the Food and Drug Administration last year marked a significant milestone in the treatment of Alzheimer’s disease. However, concerns have been raised about the safety of using this drug in individuals with Down syndrome due to a lack of data on its potential side effects in this population. Liu’s study sought to address this gap by investigating how the drug interacts with the blood vessels in the brains of individuals with Down syndrome.
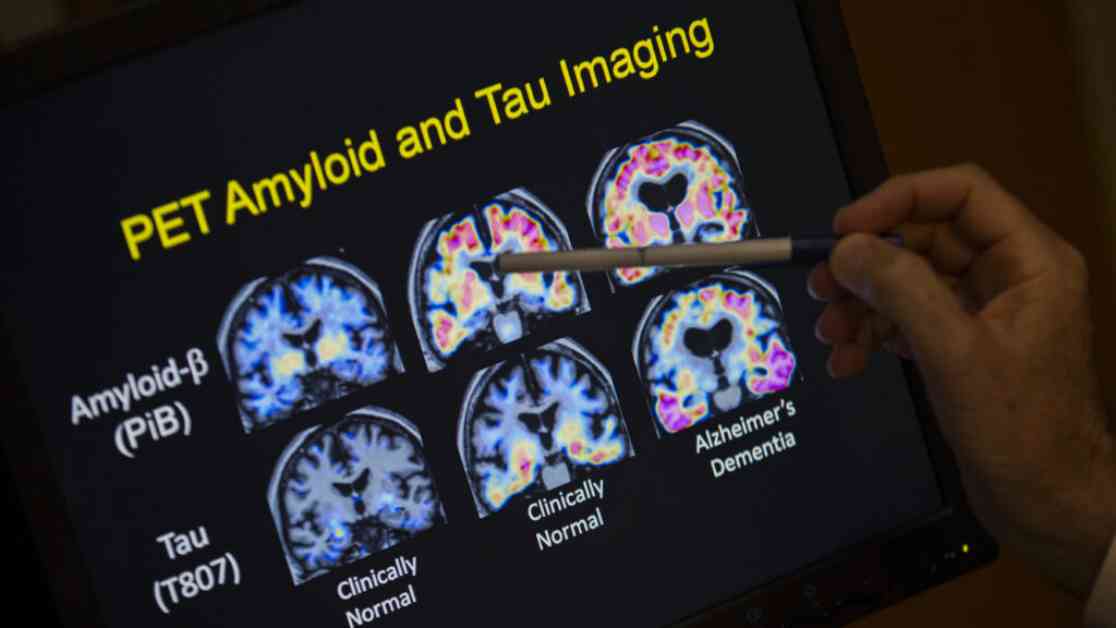
The findings of the study revealed that individuals with Down syndrome have higher levels of cerebral amyloid angiopathy (CAA), a condition characterized by the buildup of amyloid protein in the brain’s blood vessels. This increased presence of amyloid deposits in the blood vessels can weaken their walls, making them more susceptible to leakage and potentially leading to brain hemorrhages. While brain hemorrhages are rare in Alzheimer’s patients, the elevated risk factors seen in individuals with Down syndrome raise concerns about the safety of using anti-amyloid drugs in this population.
**Challenges in Clinical Trials**
Historically, individuals with Down syndrome have been excluded from clinical trials, making it difficult to assess the safety and efficacy of new treatments for conditions like Alzheimer’s disease. The lack of data on how these drugs affect people with Down syndrome has hindered progress in developing targeted therapies for this vulnerable population.
LuMind IDSC, a group advocating for better Down syndrome research, has highlighted the need for inclusion of individuals with Down syndrome in clinical trials for anti-amyloid drugs. According to LuMind IDSC president Hampus Hillerstrom, the potential benefits of these therapies, such as increased lifespan and reduced caregiving costs, must be weighed against the risks posed by potential side effects in individuals with Down syndrome.
**Implications for Future Research**
The study’s findings underscore the importance of conducting safety trials of anti-amyloid drugs in individuals with Down syndrome before widespread use in clinical settings. As research progresses, it will be crucial to monitor the effects of these drugs on real patients with Down syndrome to better understand their impact on this unique population.
Dr. Michael Rafii, a neurologist at the University of Southern California and principal investigator of an upcoming trial of donanemab in individuals with Down syndrome, emphasized the need for caution in prescribing these drugs to individuals with Down syndrome. Rafii acknowledged the elevated risk factors identified in Liu’s study and stressed the importance of conducting thorough safety trials to ensure the well-being of patients with Down syndrome.
**Conclusion**
In conclusion, the study’s findings highlight the complex relationship between anti-amyloid drugs and individuals with Down syndrome. While these drugs show promise in slowing the progression of Alzheimer’s disease, their potential side effects in individuals with Down syndrome must be carefully monitored and studied. By conducting targeted clinical trials and gathering more data on the safety and efficacy of these drugs in individuals with Down syndrome, researchers can pave the way for more personalized and effective treatments for this vulnerable population.