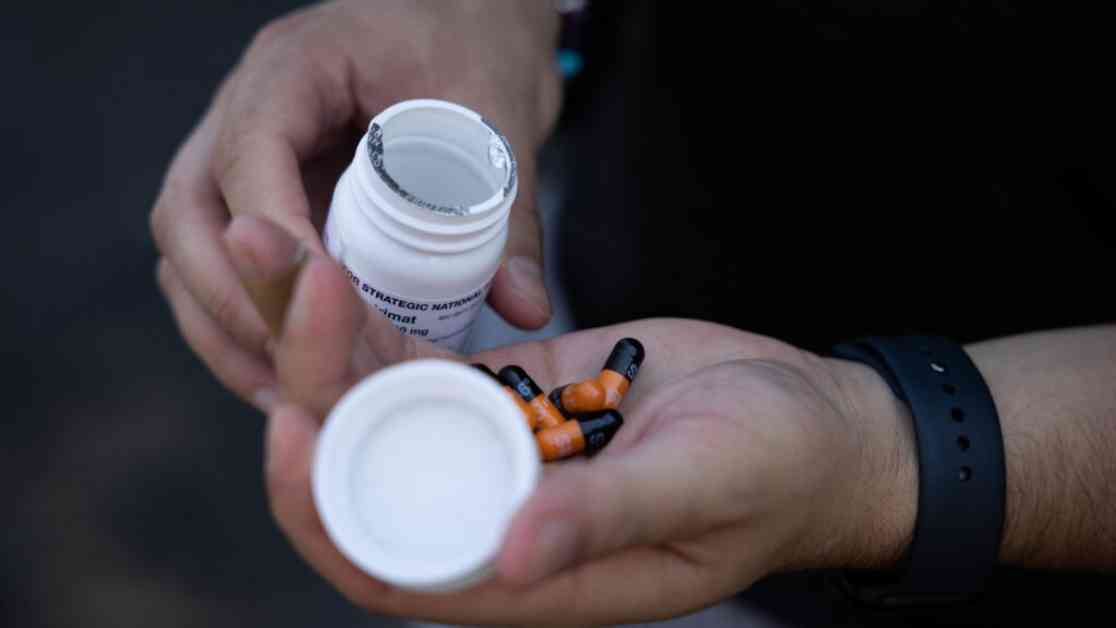
The National Institutes of Health recently conducted a study that found an antiviral drug commonly used to treat mpox was no more effective than a placebo in resolving patients’ symptoms. This news comes as a disappointment to many, as the drug, tecovirimat, also known as TPOXX, had shown promise in treating smallpox, a related virus. Despite its approval by the Food and Drug Administration in 2018 for smallpox, tecovirimat’s effectiveness in treating mpox was largely untested until now.
Background on Tecovirimat and Mpox
Tecovirimat, marketed as TPOXX, was approved by the FDA in 2018 for smallpox, a virus that has been eradicated but remains a bioterrorism concern. Due to similarities between smallpox and mpox, doctors had hoped that tecovirimat would also be effective in treating mpox. However, prior to the NIH study, data on tecovirimat’s effectiveness in treating mpox were limited to animal studies and case reports.
The Study and its Findings
The NIH co-sponsored study focused on patients in the Democratic Republic of the Congo, where mpox is endemic. Patients were randomized to receive either tecovirimat or a placebo, and both groups were required to remain in the hospital for treatment. Surprisingly, both groups saw their symptoms resolve on a similar timescale, with mortality rates below the typical rate reported in the DRC. This suggests that the high-quality supportive care provided in the hospital may have played a significant role in the patients’ outcomes, even if the drug itself did not have a noticeable impact.
Implications for Mpox Treatment
The disappointing results of the study have highlighted the need to identify other therapeutic candidates for mpox. Jeanne Marrazzo, director of the National Institute of Allergy and Infectious Diseases, emphasized the importance of continuing research on tecovirimat use in different populations affected by mpox. The study’s focus on clade I of mpox, which is known to cause more severe disease, provides valuable insights into the complexities of treating this deadly virus.
Global Concerns and Recent Outbreaks
The World Health Organization recently declared mpox a public health emergency of international concern, following a surge in cases in the Democratic Republic of Congo and other African countries. The outbreak has even spread beyond Africa, with Sweden reporting its first case in someone who had traveled to an affected region. The WHO has warned of the spread of new variants of mpox, such as clade 1b, which have been transmitted through sexual networks and other forms of contact.
Siga Technologies’ Response
Siga Technologies, the company that sells tecovirimat, stated that two subsets of patients saw “meaningful improvement” in the study: those who enrolled within seven days of symptom onset and those with severe disease. However, the company did not provide specific data or confirm if these results were statistically significant. The NIH did not highlight these subsets in its announcement, indicating that further analyses are planned. Siga Technologies’ stock experienced a significant decline following the study’s results.
Future Research and Clinical Trials
Despite the disappointing findings of the NIH study, two ongoing trials are investigating tecovirimat’s efficacy in treating clade II mpox. Early intervention appears to be crucial in achieving positive outcomes, as shown in animal studies where survival rates dropped significantly after day 5 of treatment. The results of these trials may provide valuable insights into the potential use of tecovirimat in treating different strains of mpox.
Conclusion
The NIH study’s results on tecovirimat’s efficacy in treating mpox have raised important questions about the current treatment options for this deadly virus. While the drug did not show a significant advantage over a placebo in resolving patients’ symptoms, the broader supportive care provided in the hospital appeared to play a crucial role in patient outcomes. As the global health community grapples with the ongoing mpox outbreak, further research and clinical trials will be essential in identifying new therapeutic candidates for this deadly virus.